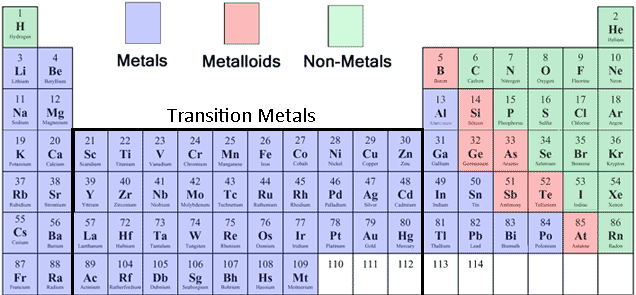
Metals and Non-Metals on the Periodic Table
Identifying metals and non-metals on the Periodic Table helps us decide the type of compound and the steps we take to name or write its formula.
A few things to note:
- In general, elements on the left of the Periodic Table are metals. On the right are non-metals.
- Hydrogen (H) is a non-metal!
- The metalloids form a dividing line between metals and non-metals.
Important Types of Compounds
| Type |
Description |
Examples |
| Ion |
Element with a Charge |
Sodium ion, Na+
Chloride ion, Cl-
Iron (III) ion, Fe3+ |
| Polyatomic Ion |
Group of Elements with a Charge |
Carbonate ion, CO32-
Nitrate ion, NO3-
Ammonium ion, NH4+ |
| Binary Ionic |
Metal + Single Non-Metal |
Sodium chloride, NaCl
Potassium sulfide, K2S
Aluminum oxide, Al2O3 |
| Ternary Ionic |
Metal + Group of Non-Metals |
Sodium nitrate, NaNO3
Calcium carbonate,CaCO3
Magnesium hydroxide Mg(OH)2 |
| Ionic with Transition Metal |
Transition Metal with Non-Metal or Polyatomic Ion |
Iron (III) chloride, FeCl3
Lead (IV) sulfate, Pb(SO4)2
Copper (I) oxide, Cu2O |
Molecular
(also called Covalent) |
Non-metal + Non-metal |
Dinitrogen pentoxide, N2O5
Carbon dioxide, CO2
Carbon monoxide, CO |
| Organic |
Compounds consisting primarily of Carbon and Hydrogen |
Methane, CH4 |
|