Finding Ionic Charge
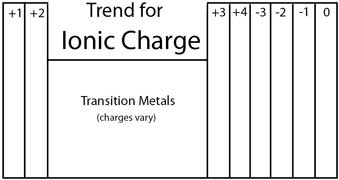
When writing formulas for ions and ionic compounds we often need to find the ionic charge. To the right is the general trend on the Periodic Table.
One way to remember is to count up to Carbon (be sure to skip the Transition Metals) and then count down using negative numbers. You should end up with zero for the Nobel Gases.
A more detailed Periodic Table with ionic charges shows where the generalization doesn't hold. Also note three Transition Metals that have only one possible charge. Ag, Cd, and Zn. These should be memorized.
For high school chemistry just knowing the trend (the top Periodic Table) may be sufficient. For more advance courses knowing the trend and then learning the exceptions is necessary.
|