 |
Chemical Bonding: Valence Electrons |
|
Lewis structures have a major flaw. For many structures (O3, NO3-, etc) drawing a single structure won't show the actual distribution of electrons between atoms. For these structures experimental data tells us a single Lewis structure isn't an accurate picture of the molecule as found in the real world. To get around this we draw resonance structures. For example, for O3 we draw: 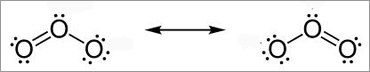
The actual structure is an average of the two structures. Experimental data tells us that the bonds between the Oxygen atom are actually one and a half (1 1/2) and not double bonds. What you need to know:
|
|