 |
Chemical Bonding: Calculating Formal Charges |
|
Sometimes we're not sure if we've drawn the Lewis structure correctly. Other times there may be more than one way to draw a Lewis structure. Formal Charges let us check to be sure we have the best structure*. 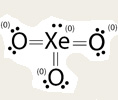
A few things to note:
Video: Calculating Formal Charges
*The best structure is the most common form of the molecule. In a given sample the majority of the molecules will have a Lewis structure where the formal charges are closer to zero (with the exception of ions). |
|