 |
Chemical Bonding: Valence Electrons |
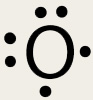
To be successful in chemistry you must be able to find the number of valence electrons for individual elements. A few things to note:
Video: Finding Valence Electrons on the Periodic Table Video: Finding the Number of Valence Electrons for a Molecule |
|