 |
Chemical Bonding: Polarity |
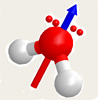
Many molecules have a negative side (pole) and a positive (pole). These are called polar molecules. Nonpolar molecules have a uniform charge (no + or – poles). Polarity helps use understand how molecules will interact with each other. For example, the strands of your DNA are held together because polar molecules.
Video: Polarity (from IssacsTeach) |
|